🧬 Biotechnology Investment Snapshot
| 📌 Key Detail | 📊 Information |
|---|---|
| 🔬 Maturity Stage | Prototype / Preclinical |
| 💶 Pre-Money Valuation | €5,000,000 |
| 📅 Estimated Exit | 2030 |
| 🧪 Sector | Biotechnology |
| 🎯 Equity Offered | 15% |
| 💰 Minimum Investment | €1,000 |
| 📍 Location | Limoges 🇫🇷 |
| 📈 Investment Type | Equity |
| 🧾 Tax Deduction | Eligible |
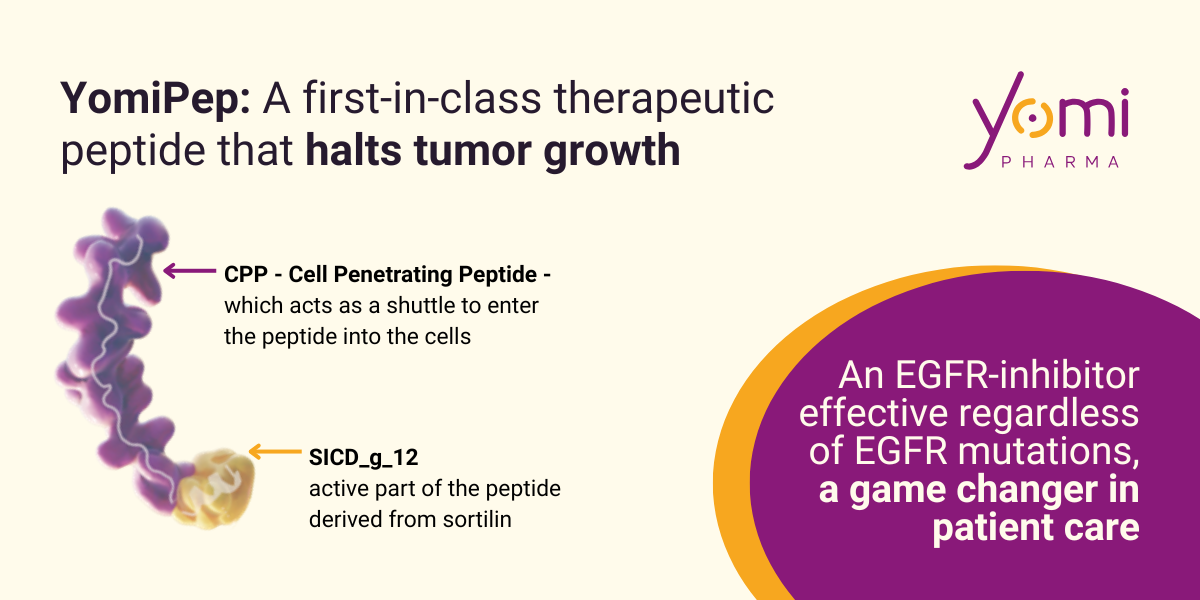
Yomi Pharma is a biotech spin-off from the University of Limoges that is developing a targeted therapy for resistant forms of lung cancer. Its drug candidate, YomiPep, is a patented first-in-class peptide that acts in a completely novel way to inhibit the EGFR receptor, a key target in one of the most common lung cancer subtypes found in patients.
While all current anti-EGFR therapies systematically fail due to the emergence of resistance, YomiPep changes the game by acting at the heart of the tumor cell. For patients who are left with only palliative chemotherapy, this is new hope where there was none.
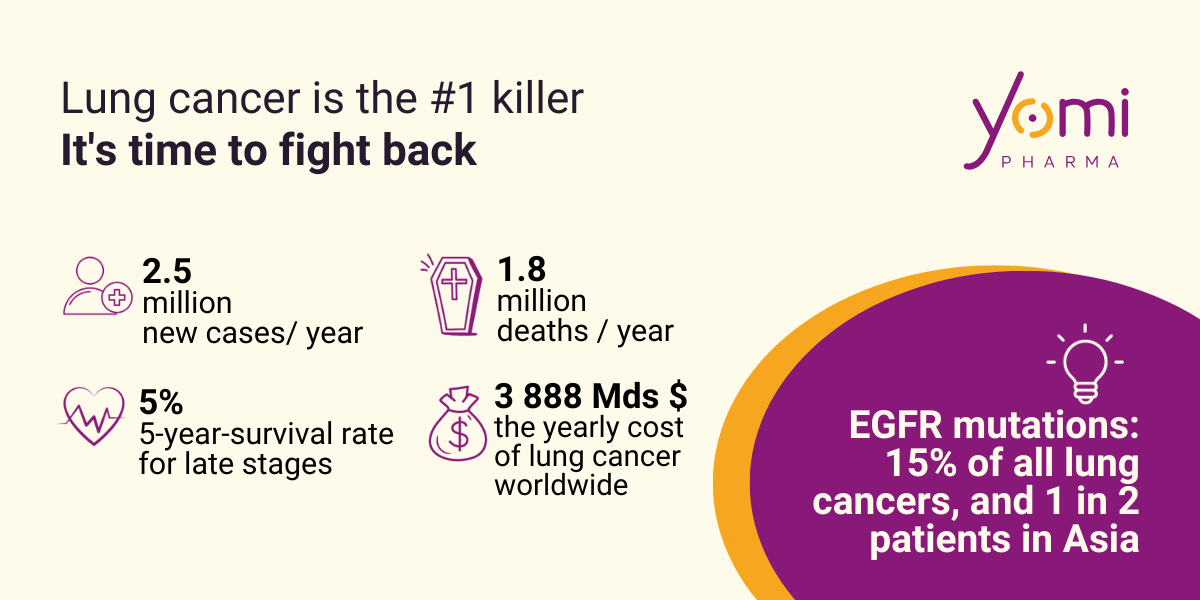
Lung cancer: a global health emergency
Lung cancer is the deadliest of all cancers, responsible for more than 1.8 million deaths and 2.5 million new cases each year. Its economic impact is colossal: it alone accounts for 15% of all cancer-related economic costs in Europe (€18.8 billion). It frequently involves alterations in key genes such as EGFR.
EGFR receptor mutations are most common in lung cancer
EGFR is a membrane receptor that controls cell growth. The appearance of mutations in this gene promotes tumor progression. Approximately 15% of Western patients (and nearly 1 in 2 patients in Asia) with lung cancer have this type of EGFR mutation, making this onco-receptor the preferred target of current therapies. And contrary to popular belief, patients with this type of lung cancer are predominantly women, young, and have never smoked.
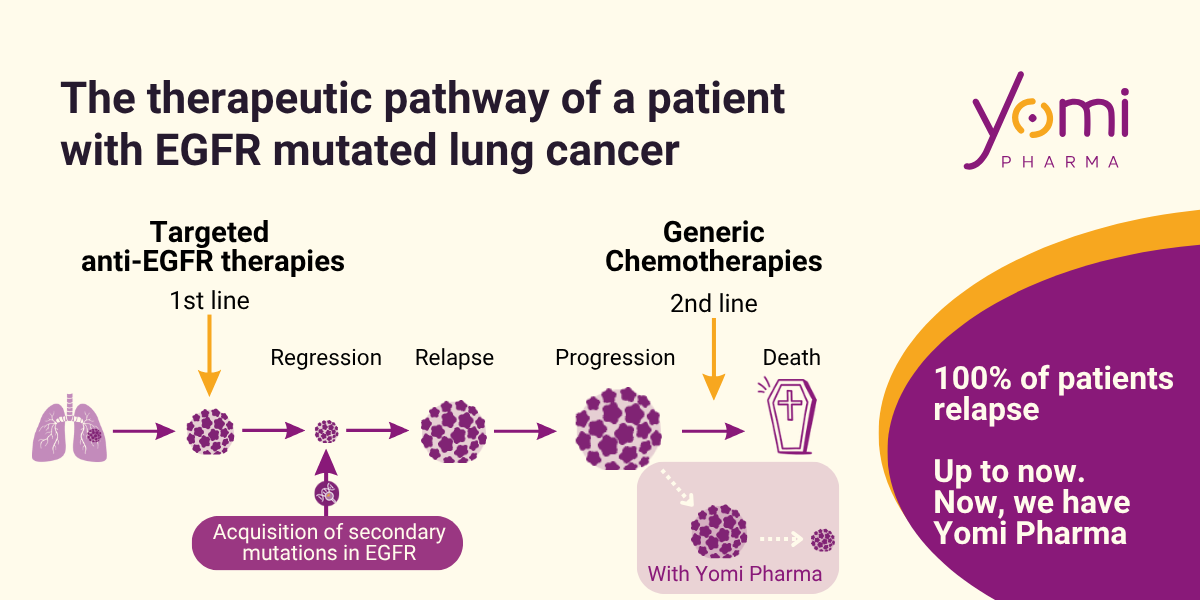
Targeted anti-EGFR therapies: progress on survival but inexorable relapse in 100% of patients
Targeted anti-EGFR therapies have led to major advances, doubling progression-free survival (to 19 months today). However, cancer always ends up resisting these treatments, often by acquiring new mutations in the EGFR, or by circumventing the main pathway of action of these drugs.
Sortilin: a protein with anti-tumor properties in lung cancer
Sortilin is a protein whose expression level is associated with a good prognosis in lung cancer: in patients who express a high level of sortilin expression, their survival is doubled compared to those who express very little.
YomiPep: a novel, intracellular approach effective against all EGFR mutations
Yomi Pharma is harnessing the natural anti-tumor properties of sortilin by developing YomiPep, an innovative peptide that replicates its action. It acts directly in the nucleus of tumor cells by blocking the oncogenic activity of EGFR at the gene transcription level. Thanks to its unique mechanism of action, YomiPep is active regardless of EGFR mutation status, including rare mutations.
A clear clinical positioning associated with a strong addressable market
Lung cancer is one of the most valuable segments in oncology, driven by increasing prevalence and strong industrial partnership activity from the preclinical phases (15 deals over the last 10 years). The global market for targeted anti-EGFR therapies in lung cancer is estimated at €14 billion, and is expected to double by 2032. With sales exceeding $6.6 billion per year, osimertinib (Tagrisso TM , AstraZeneca), a first-line anti-EGFR treatment, illustrates the scale of the market. Its patent expiring in 2032 opens a strategic window for a new disruptive player like YomiPep.
YomiPep’s positioning was validated by Professor Nicolas Girard (pulmonary oncologist at the Curie Institute), an expert in EGFR mutated lung cancer and member of the Yomi Pharma board, as well as several oncologists contacted as part of a market study conducted by the firm In Extenso Innovation Croissance.
Two strategic segments with strong medical and commercial impact
YomiPep fits into current treatment lines:
- In 2nd line of treatment after failure of targeted anti-EGFR therapies, for common mutations (e.g. L858R, Del19): 28,000 patients/year and a turnover potential of $3.4 billion
- In first- line treatment for rare EGFR mutations, currently under-treated: 11,000 patients/year and a potential turnover of $1.1 billion
An expert team and a renowned medical board
Led by Camille Granet, entrepreneur and researcher at the origin of the scientific discovery, Yomi Pharma brings together an experienced multidisciplinary team combining scientific expertise, preclinical development and biotech strategy.
800,000 euros sought in seed
Yomi Pharma is raising €800k today, of which €335k has already been secured from Business Angels, to continue preclinical research, work on the formulation of the peptide and structure its team, with the objective of entering the regulatory preclinical phase in 2027.
With a current valuation of €5 million and a potential exit valuation of up to €175 million within 5 years, this is an opportunity for investors to join an early stage deal with a potential ROI of up to x21.
Why is Capital Cell investing in this company?
Between the 1970s and 1980s, the discovery of the EGFR receptor as a regulator of cell proliferation earned Stanley Cohen a Nobel Prize. In the 2000s, its key role in cancer was confirmed, giving rise to four generations of treatments targeting its membrane-bound form. The result: doubled response rates, but inevitable relapse, regardless of the mutation.
Faced with this unmet medical need, Yomi is proposing a paradigm shift with sortilin: a protein associated with a better prognosis because it allows direct intervention in the cell nucleus, where EGFR activates the genes responsible for tumor survival and proliferation.
Yomi has developed YomiPep, a peptide derived from sortilin, capable of penetrating the nucleus to block the action of EGFR on its targets. The result: proliferation is stopped, and the cell death signal is reactivated. In animal models, YomiPep reduces tumor size by 50%, without toxicity. The molecule is firmly protected by patent, with a clear regulatory strategy: first in 2nd line, to treat patients who relapse after resistance (which happens in 100% of patients), and demonstrate its efficacy. Then, in 1st line, in combination with other therapies — and in parallel, as monotherapy in 11,000 patients/year carrying rare EGFR mutations, with no other option available.
The market is growing rapidly, driven by rising prevalence and treatments such as Tagrisso (osimertinib), AstraZeneca’s blockbuster ($6.6 billion in 2024), whose patents expire in 2032. While no therapy is able to prevent relapses, and the market will soon see changes in IP, Yomi is positioning itself with a completely new approach. Valued at €5 million, with the potential to increase 20-fold in five years, this is a highly leveraged investment opportunity—at the right time.
Minimum investment: 1.000€
Type of exit expected: Acquisition by a pharmaceutical company
Drag-along rights
Tag-along rights
Preferential liquidation right
Anti-dilution law
Capital Cell's right to attend and represent investors at the Shareholder Meeting
Tax deductions
Subscribing to the capital of a company, such as a JEIR, allows you to benefit from an income tax reduction (IR-PME) equivalent to 50% of the amounts invested, subject to meeting certain conditions. The subscriber must be a natural person, i.e., an individual or sole trader, domiciled for tax purposes in France, and undertake to hold the securities received in exchange for the subscription (shares or stocks) for a minimum period of five years. The amount of payments taken into account for the calculation of this tax reduction is limited to €50,000 for a single, widowed, or divorced person, and to €100,000 for a married or civil partnership couple subject to joint taxation. For more information, you can visit the website: entreprendre.service-public.fr.
Shareholder reporting obligations every 3 months
Main risks
Yomi is still in its very early stages, which implies significant uncertainties related to clinical and regulatory development. Furthermore, the EGFR target is well known and extensively studied, with strong competition already established in the market. Several attempts to target this pathway have not produced sustainable results, which underscores the scientific and commercial difficulty of the field.